Assistant Investigator
Other positions:
Associate Professor of Microbiology, Department of Environmental, Biological and Pharmaceutical Sciences and Technologies,University Luigi Vanvitelli, Caserta, Italy
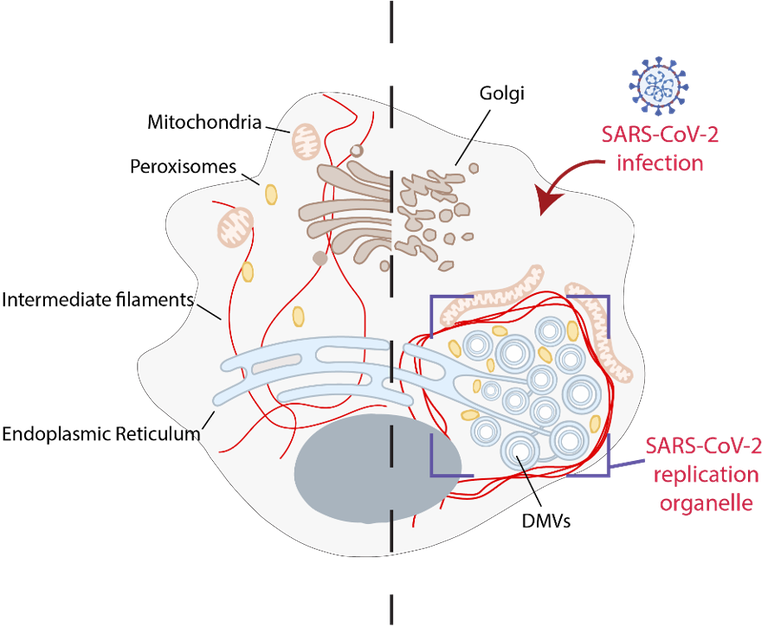
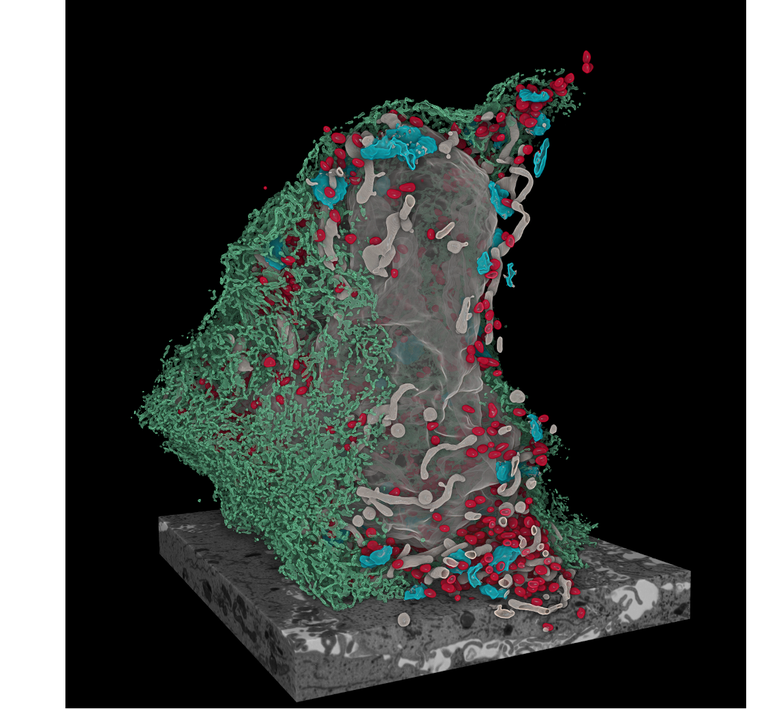
Mirko Cortese studied Biotechnology at the University of Naples “Federico II”. After his graduation in 2008, he moved to GSK Vaccines (ex Novartis Vaccines and Diagnostics) in Siena for a post-graduate internship in the field of vaccine development. There he pursues his doctorate studies in collaboration with the University of Bologna on the implementation of “reverse vaccinology” to identify novel candidate antigens to develop a vaccine against human Cytomegalovirus. After his PhD in 2013, he moved to Germany for a post-doc in the Department of Infectious Diseases at the University of Heidelberg. There he studied the mechanisms of replication of positive-sense single-stranded RNA (+ssRNA) viruses and how viral infections rewire the host cell to create an environment conducive to viral replication and spread. He investigated two clinically important human pathogens, dengue virus and Zika virus, that can cause severe diseases. He described the ultrastructure of the viral replication organelle, a specialized intracellular compartment formed during +ssRNA virus infection in which viral genome replication takes place. More recently, he characterized the morphological alterations induced during the SARS-CoV-2 replication cycle and described how viral replication affect cellular organelles morphology and function thus contributing to viral cytopathogenicity.

- A Versatile Reporter System to Monitor Virus Infected Cells and Its Application to Dengue Virus and SARS-CoV-2. Journal of Virology, 2020
- Integrative Imaging Reveals SARS-CoV-2-Induced Reshaping of Subcellular Morphologies. Cell Host & Microbe, 2020
- SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nature Communications, 2020
- Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature, 2020
- Ultrastructural Characterization of Zika Virus Replication Factories. Cell Rep, 2017
Complete List of Published Work here
Quote
I’ve recently joined the TIGEM faculty and I have found awesome scientists, great infrastructure, and excellent support. I cannot wait to start this new exciting adventure.
Additional Funding
- FTH - FONDAZIONE HUMAN TECHNOPOLE - AAV vectors in the Human Central Nervous System (2023)










