Innovative AAV-HITI Technology for Long-term Gene Therapy
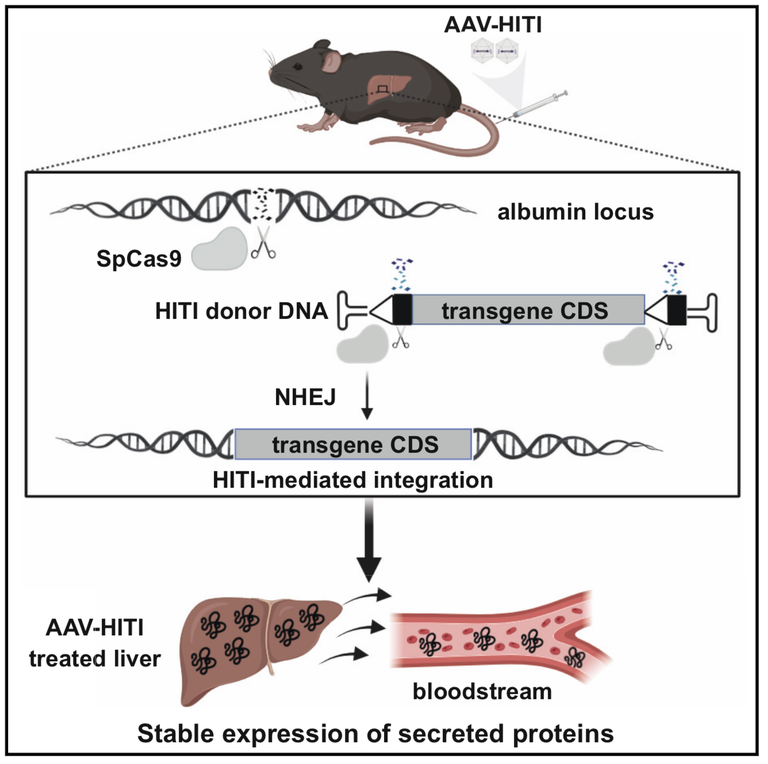
We are excited to announce the publication of Alberto Auricchio's new article in Cell Reports Medicine, detailing our proprietary Liver-directed adeno-associated viral (AAV) vector-mediated homology-independent targeted integration (AAV-HITI) technology developed using CRISPR-Cas9. This groundbreaking approach targets the highly transcribed albumin locus in the liver, achieving productive integration in approximately 15% of hepatocytes and resulting in therapeutic protein levels in mouse models of inherited diseases. Our research demonstrates significant therapeutic benefits without major off-target effects or toxicity, supporting AAV-HITI as a safe and effective gene therapy strategy for both newborn and adult patients.Innovative HITI Technology for Sustained Gene Therapy
In the quest to provide lasting therapeutic solutions for inherited diseases, our team has developed a groundbreaking technology: Liver-directed adeno-associated viral (AAV) vector-mediated homology-independent targeted integration (AAV-HITI) using CRISPR-Cas9. This proprietary technology is currently under investigation for its potential to offer sustained transgene expression following neonatal treatment.
Targeting the Albumin Locus
Our research focuses on targeting the 3' end of the albumin locus, a highly transcribed region in the liver. This approach has shown promising results, with productive integration occurring in approximately 15% of mouse hepatocytes. This level of integration is sufficient to achieve therapeutic levels of systemic proteins, as demonstrated in two different mouse models of inherited diseases.
Mechanism and Efficiency
The AAV-HITI technology leverages the efficiency of the CRISPR-Cas9 system to facilitate the integration of full-length HITI donor DNA into the target site. Upon nuclease cleavage, the donor DNA is preferentially integrated, ensuring stable and sustained expression of the therapeutic gene. Importantly, our studies have revealed no significant chromosomal rearrangements or insertions/deletions at off-target sites, indicating a high degree of specificity and safety.
Long-term Safety and Efficacy
Our long-term follow-up studies, extending up to one year, have shown no evidence of hepatocellular carcinoma, underscoring the safety of the AAV-HITI approach. Additionally, we have demonstrated that the technology is effective at vector doses considered safe for human application, providing therapeutic efficacy in both adult and neonatal liver.
Addressing Challenges in Gene Therapy
Adeno-associated viral (AAV) vectors are widely regarded as the most effective tool for in vivo gene therapy due to their safety, efficacy, and capacity for long-term transgene expression. In liver-directed gene therapy, a single intravenous administration of AAV can convert hepatocytes into a factory for sustained protein secretion, offering long-term therapeutic effects in both preclinical and clinical settings.
However, the non-integrative nature of traditional AAV genomes limits their use in neonatal treatments. This is particularly problematic for early-onset inborn errors of metabolism, where liver growth leads to AAV genome loss over time, diminishing therapeutic efficacy. Our recently completed phase 1/2 clinical trial for mucopolysaccharidosis type VI (MPS VI) excluded patients under four years of age for this reason (ClinicalTrials.gov, NCT03173521). Moreover, the immune response against AAV capsid proteins can prevent repeat administrations.
Advancements in Genome Editing
To overcome these challenges, genome editing technologies, especially those involving CRISPR-Cas nucleases, are being extensively explored. Homology-independent targeted integration (HITI) has emerged as a promising strategy to achieve stable gene expression. By targeting the mouse albumin (mAlb) locus, which integrates via non-homologous end-joining (NHEJ), we aim to provide stable and enduring gene therapy solutions.
Conclusion
Our data support the development of AAV-HITI as a viable and effective liver-directed gene therapy strategy. By addressing the limitations of traditional AAV-based therapies, our approach holds the potential to transform the treatment landscape for various inherited diseases, offering hope for lasting therapeutic benefits from a single, early-life intervention. As we continue to advance this technology, we remain committed to ensuring its safety, efficacy, and accessibility for patients in need.
Link to the article here
