Toward Common Solutions for Rare Retinal Diseases: Commenting on miRNA-Based Therapeutic Strategies for IRDs
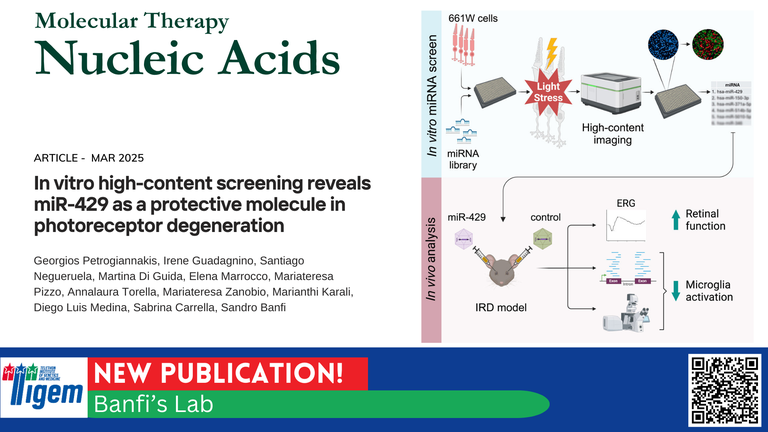
In a compelling commentary published in Molecular Therapy – Nucleic Acids, Jan Wijnholds discusses the promising results of a recent study by Petrogiannakis et al., led by Sandro Banfi’s group at TIGEM, that introduces miR-429 augmentation as a potential generic gene therapy strategy for inherited retinal diseases (IRDs). Their work significantly contributes to an evolving and urgent therapeutic landscape that seeks mutation-independent approaches to combat photoreceptor (PR) degeneration.
Inherited retinal diseases represent one of the most genetically heterogeneous classes of disorders, with over 318 causative genes identified to date. This staggering diversity makes gene-specific therapies—like gene replacement or editing—logistically and economically challenging to scale. After the approval of Luxturna in 2017 for RPE65-associated retinal dystrophy, the pipeline for additional gene therapies has been slow, leaving the majority of IRD patients still without viable treatments.
Banfi’s group takes a different path. Using high-content imaging, they screened over 1,200 microRNAs (miRNAs) and identified miR-429 as a potent protector against PR degeneration. Delivered via AAV8 vectors, miR-429 preserved PR function and reduced inflammation in the RhoP23H/+ mouse model of retinitis pigmentosa. This strategy not only complements previous efforts targeting PR metabolism (like RdCVF and PHD2 downregulation), but opens a new chapter for miRNA-based neuroprotection in IRDs.
miRNAs: Small RNAs, Big Impact
The therapeutic promise of miRNAs goes beyond their small size. These 18–24 nucleotide non-coding RNAs orchestrate complex gene networks by silencing multiple mRNA targets. They act as molecular regulators of stress responses, apoptosis, metabolism, and immune pathways—processes commonly dysregulated across many forms of retinal degeneration.
Banfi’s work builds on previous successes with miR-204 and miR-181a/b, showcasing the potential of both overexpression and inhibition strategies. What makes miRNAs potentially appealing therapeutic targets is their broad mechanism of action, capable of targeting converging pathological pathways shared across genotypes.
The recent Nobel Prize awarded for the discovery of miRNAs further validates research on miRNAs as a transformative field for medicine and adds visibility and credibility to their therapeutic exploitation.
The Power of Gene-Independent Strategies
Perhaps the most powerful implication of this work lies in its gene-independent framework. Developing a single therapy for a rare condition affecting a handful of patients is financially unsustainable under traditional models. However, a "common therapeutic denominator"—like miR-429—that targets shared degeneration pathways could vastly expand the patient population eligible for treatment. This would not only make drug development more efficient but could also reduce costs, enhance commercial viability, and accelerate clinical translation.
Other promising generic (gene agnostic) approaches include:
- AAV-mediated augmentation of RdCVF and RdCVFL to support cone metabolism;
- CRISPR-based downregulation of PHD2, enhancing hypoxic responses to protect PRs.
These approaches, together with miRNA augmentation strategies like miR-429, represent a new therapeutic class—not bound to the mutation but to the mechanism.
Toward Clinical Translation
The road ahead involves validating these findings in large animal models and human-derived retinal organoids. The challenge remains to unravel the precise mechanism of action of miR-429 and confirm its evolutionary conservation in human photoreceptors. But if successful, these studies may establish miRNA-based AAV vectors as the next wave of IRD therapeutics.
As Wijnholds concludes, miR-429 now joins a short but urgent list of mutation-independent interventions capable of delaying or even preventing photoreceptor loss in IRDs. Thanks to the pioneering work of researchers like Sandro Banfi and his team at TIGEM, the field is moving closer to common solutions for rare diseases—solutions that could transform the therapeutic landscape and restore hope for thousands of patients still awaiting their cure.
